Answer:
V = 150.1 mL
Step-by-step explanation:
Hello!
In this case, since the formula for the calculation of density is:
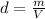
Thus, since we need to compute the volume of 131.9 g of benzene, we solve for V in the aforementioned equation to get:
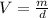
Therefore, we plug in the given mass and density to obtain:
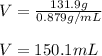
Best regards!