Answer:
The mass of the titanium ring is 1.8 grams.
Explanation:
Given : The density of titanium is 4.5 g/cm³ . A titanium ring has a volume of 0.4 cm³.
We have to find the mass of the titanium ring.
Using formula for density
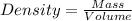
Rearranging for mass, we have
Multiply both side by Volume, We have,
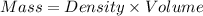
Given: Density = 4.5 g /cm³ and Volume = 0.4 cm³
Thus,
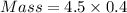
Smplify, we have,
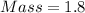 grams
grams
Thus, The mass of the titanium ring is 1.8 grams.