Answer:
-14,240.8 kJ of heat is produced by the complete combustion of 284 grams of methane.
Step-by-step explanation:
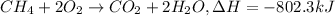
Mass of methane = 284 g
Moles of methane =

According to chemical reaction, combustion of 1 mole of methane gives 802 kilo Joules of heat , then 17.75 moles of methane of on combustion will give :
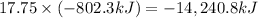
-14,240.8 kJ of heat is produced by the complete combustion of 284 grams of methane.