Answer:
83.99
Explanations:
The formula for sodium bicarbonate is given as NaHCO₃
To calculate the molar mass, we need to get the molar mass of each element first.
Molar mass of sodium element (Na) = 22.98g/mol
• Molar mass of ,Hydrogen, element = 1.00g/mol
• Molar mass of ,Carbon, element = 12.01g/mol
• Molar mass of, Oxygen, element = 16.00g/mol
Determine the molar mass of the compound
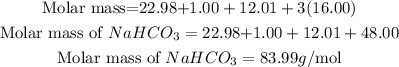
Hence the molar mass of sodium bicarbonate to two decimal places is 83.99