Answer: Option (a) is the correct answer.
Step-by-step explanation:
A lithium atom has atomic number 3 and its electronic distribution is 2, 1. Therefore, it easily loses one electron and results in the formation of
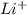 which is known as lithium ion.
which is known as lithium ion.
Whereas a nitrate ion has a chemical formula as
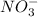 .
.
Since, charge on both lithium and nitrate ion is same in number. Therefore, when both these ions combine together then it results in the formation of
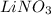 compound.
compound.
Name of this compound is lithium nitrate.
Thus, we can conclude that when a lithium ion and nitrate ion combine, the result is
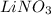 .
.