In the exercise, we are asked to find the number of molecules having the mass of CaSO4. The number of molecules also called formula units is a relation found by a scientist called Avogadro. This ratio tells us that in one mole of any substance we will always find 6.022 x10^23 molecules or formula units.
Now, the first thing to do is to calculate how many moles are in 15.255 grams of CaSO4. For that they use the molar mass of the molecule and apply the next equation:
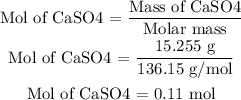
After having the number of moles now apply the Avogadro ratio to calculate the number of molecules or formula units.
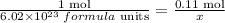
Clear x,
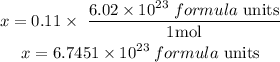
They do everything in one step. Calculate the number of moles and immediately apply Avogadro.