Answer : The amount of zinc obtained can be 396.9 tons.
Explanation :
First we have to calculate the amount zinc carbonate in 976 tons.
Amount of zinc carbonate =
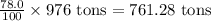
Now we have to calculate the percentage of zinc in zinc carbonate.
As we know that,
Atomic mass of zinc = 65.38 g
Atomic mass of zinc carbonate = 125.38 g
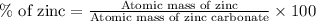
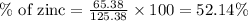
Now we have to calculate the amount of zinc in 761.28 tons.
Amount of zinc =
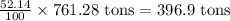
Therefore, the amount of zinc obtained can be 396.9 tons.