Answer:
![Rate=k[Z]^3](https://img.qammunity.org/2018/formulas/chemistry/high-school/x4eddrr7h83jtnc1yi6lqvzonxyzzyornn.png)
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
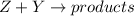
![Rate=k[Z]^x[Y]^y](https://img.qammunity.org/2018/formulas/chemistry/high-school/uos31drzom0rv3zqru4o0hzemmha3c5azp.png)
k= rate constant
x = order with respect to Z = 3
y = order with respect to Y = 0
n = x+y = Total order
Thus rate law is written as:
![Rate=k[Z]^3[Y]^0](https://img.qammunity.org/2018/formulas/chemistry/high-school/tmrkllvk9uwh3pvwp4ok8xtwj09j2zr67t.png) or
or
![Rate=k[Z]^3](https://img.qammunity.org/2018/formulas/chemistry/high-school/x4eddrr7h83jtnc1yi6lqvzonxyzzyornn.png)