First, we need to state the chemical equation for the combustion of PH3

And the mass of PH3 is 17.0 grams and we need to know the moles.
In the periodic table, the atomic mass of the P (phosphorus) is 31 and the atomic mass of the H (hydrogen) is 1.
So, you sum the mass of P to the mass of H multiplied by 3 and you obtain this:
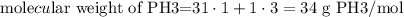
With this data, we can search the moles of PH3: