ANSWER
The final temperature of the gas is 380K
Step-by-step explanation
Given that;
The initial pressure of the gas is 258kPa
The initial temperature of the gas is 3 degrees Celcius
The final pressure of the gas is 355kPa
To find the final pressure of the gas, follow the steps below
In the given data, the volume of the container is fixed. Hence, the process is called isochoric.
Step 1: Write the gas law at constant volume
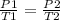
Step 2: Convert the temperature to kelvin
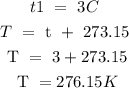
Step 3: Substitute the given data into the formula in step 1

Hence, the final temperature of the gas is 380K