Answer:
Step-by-step explanation:
Hello!
In this case, since HCl and Pt react according to the following chemical equation:
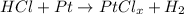
Whereas PtClx is the compound containing Pt and Cl; thus, since 1.018 g out of 1.778 g correspond to Pt and therefore 0.760 g to chlorine, so we determine the empirical formula of this compound by firstly computing the moles of each element:
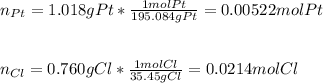
Now, we divide the each moles by those of Pt as the fewest ones in order to compute their subscripts in the empirical formula:
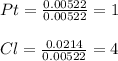
Thus, the required formula is:
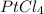
Best regards!