Answer: D) it doubles in volume
Step-by-step explanation: This can be explained by using Charle's law.
Charles' Law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
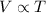 (At constant pressure and number of moles)
(At constant pressure and number of moles)
Thus when temperature is doubled at constant pressure and constant number of moles, the volume also doubles.