Answer : Ammonia has molecular formula as
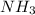
It has a central atom as Nitrogen bonded with 3 molecules of hydrogen around it, Nitrogen has atomic number 7
The electronic configuration of N is
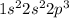
The number of valence electrons are 5 in the outermost shell of nitrogen.
According to the octet rule, it needs 3 electrons to be an octet.
So, the valency of N is 3. And, Hydrogen has the valency as 1.
Therefore, three hydrogen are needed to be a stable compound of
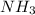 .
.
The lewis structure of
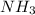 is attached in the answer.
is attached in the answer.
In the attached figure, the electrons of N are shown in dot and the electrons of H are shown in x.