Answer:
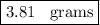
Step-by-step explanation:
First consider the mol to mol ratio, the mol of a substance is simply the count of atoms in respect to avagadros number (approx. 6.02 × 10²³ molecules) in the period table. 1 mol of an element is simply it's mass count in the periodic table.