Answer : The correct option is, -6.32 KJ
Solution : Given,
Mass of methanol = 64 g
Molar mass of methanol = 32 g/mole
Heat of fusion = 3.16 KJ/mole
First we have to calculate the moles of methanol.
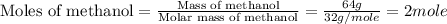
Moles of methanol = 2 moles
Now we have to calculate the heat of solidification.
As, 1 mole of methanol contains heat = 3.16 KJ
So, 2 mole of methanol contains heat =
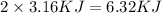
The heat of solidification is, -6.32 KJ. The negative sign indicate the heat released in the system.
Therefore, the heat of solidification is, -6.32 KJ