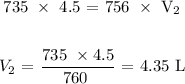Answer:
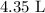
Step-by-step explanation:
Here, we want to get the volume occupied at the new temperature
According to Boyle's law, volume and pressure are inversely proportional
Thus, mathematically:
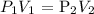
P1 is the initial pressure which is 735 mmHg
V1 is the initial volume which is 4.5 L
P2 is the final pressure which is 1 atm( 760 mmHg)
V2 is the final volume that we want to calculate
Substituting the values, we have it that: