Answer:
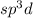 orbital.
orbital.
Step-by-step explanation:
Valence bond theory is the chemical bonding theory which explains the chemical bond between two atoms. Both of the two atoms shares each of the unpaired electron to form a hybrid orbital and together to form a bond.
According to the valence bond theory, P-Cl bond forms by the overlap of a phosphorus
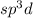 orbital with chlorine 3p orbital to form five sigma bond (P-Cl). The five
orbital with chlorine 3p orbital to form five sigma bond (P-Cl). The five
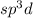 orbital are single occupied.
orbital are single occupied.
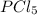 has a trigonal bipyramidal structure, and the bond angle is 90 and 120 degree. The original atomic orbital are no longer exist.
has a trigonal bipyramidal structure, and the bond angle is 90 and 120 degree. The original atomic orbital are no longer exist.