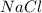Answer:
Covalent bonds are formed by sharing of electrons between non metals.
A polar covalent bond is defined as the bond which is formed when there is a difference of electronegativities between the atoms. It is also defined as the bond which is formed due to the unequal sharing of electrons between the atoms. example:
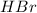
Non-polar covalent bond is defined as the bond which is formed when there is no difference of electronegativities between the atoms. It is also defined as the bond which is formed due to the equal sharing of electrons between the atoms. Example:
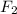
Ionic bonds are formed by transfer of electrons between metal and non metals. Example: