Answer: The correct answer is Option B.
Explanation: Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
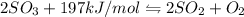
This is a type of Endothermic reaction because heat is absorbed in the reaction.
For the given options:
Option A: Decrease the temperature
If the temperature is increased, so according to the Le-Chatlier's principle , the equilibrium will shift in the direction where increase in temperature occurs. As, this is an endothermic reaction, forward reaction will decrease the temperature. Hence, the equilibrium will shift in the left direction.
Option B: Increase the volume
If the volume of the container is increased, the pressure will decrease according to Boyle's Law. Now, according to the Le-Chatlier's principle, the equilibrium will shift in the direction where increase in pressure is taking place. As the number of moles of gas molecules is greater at the product side. So, the equilibrium will shift in the right direction.
Option C: Add more
 gas
gas
If the concentration of
 that is the product is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of
that is the product is increased, so according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease of concentration of
 takes place. Therefore, the equilibrium will shift in the left direction.
takes place. Therefore, the equilibrium will shift in the left direction.
Option D: Add a catalyst
Role of catalyst is to attain the equilibrium quickly without disturbing the state of equilibrium. Hence, addition of catalyst will not change the equilibrium of the reaction.
From the above explanation, we can easily say that the correct answer is Option B.