Answer:The correct order is:
ethane, pentane, heptane, octane
Step-by-step explanation:
The boiling point of the alkanes increases with increase with an molecular mass that is higher the number of carbon atoms in the alkane higher will be the boiling point of the alkane.
With increase in molecular area the surface area and association of an alkane also increases.
Ethane =
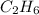 , 2 carbon atoms.
, 2 carbon atoms.
Pentane =
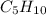 ,5 carbon atoms.
,5 carbon atoms.
Heptane =
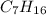 ,7 carbon atoms.
,7 carbon atoms.
Octane =
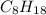 ,8 carbon atoms.
,8 carbon atoms.
Straight-chain alkanes from lowest to highest boiling point:
ethane<pentane<heptane< octane