Answer : The number of unpaired electrons present on iron in iron(II) sulfide are, 4
Explanation :
The given compound is, FeS
In this compound, iron is in (+2) oxidation state and sulfur is in (-2) oxidation state.
As we know that, iron is a d-block element and the atomic number of iron is 26. That means, there are 26 number of electrons.
The electronic configuration of iron is:
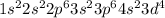
The electronic configuration of Fe²⁺ will be:
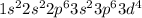
Thus, there are 4 unpaired electrons present on iron in iron(II) sulfide.