Given:
Mass of water, m = 3.0 kg
Room temperature, T1 = 20°C
Let's find the amount of energy necessary to heat the water from 20°C to its boiling point.
Where:
Boiling Point of water, T2 = 100 °C
Apply the formula:
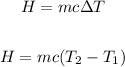
Where:
• H is the required energy
,
• m is the mass
,
• c is the specific heat capacity of water = 1.0 kcal/kg. °C
,
• T2 is the final temperature = 100 °C
,
• T1 = 20°C
Thus, we have:
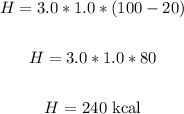
Therefore, the required energy is 240 kcal.
• (b). If electrical energy were used, how much would this cost at 27¢ per kWh?
Where:
1 kcal = 0.00116 kWh
To find the cost at 27¢ per kWh, we have:
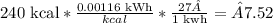
Therefore, the cost will be 7.52¢
ANSWER:
• (a). 240 kcal
• (b). 7.52,¢