Answer: The correct answer is Option D.
Step-by-step explanation:
Limiting reagent is defined as the reagent which limits the formation of product and is present in less quantity as a reactant.
Excess reagent is defined as the reagent which is present in excess as a reactant.
To calculate the number of moles, we use the equation:
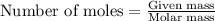
Given mass of carbon dioxide = 42 g
Molar mass of carbon dioxide = 44 g/mol
Putting values in above equation, we get:
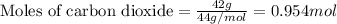
Given mass of KOH = 99.9 g
Molar mass of KOH = 56.1 g/mol
Putting values in above equation, we get:
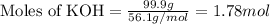
For the given chemical reaction:
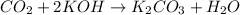
By Stoichiometry of the reaction:
2 mole of potassium hydroxide reacts with 1 mole of carbon dioxide.
So, 1.78 moles of KOH will react with =
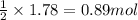 of carbon dioxide.
of carbon dioxide.
As, the required amount of carbon dioixde is less than the given amount. Thus, it is considered as an excess reagent.
Potassium hydroxide is considered as a limiting reagent becase it limits the formation of products.
Thus, the correct answer is option D.