Answer:
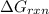 for the given reaction is 130.19kJ/mol
for the given reaction is 130.19kJ/mol
Explanation:
To Calculate the
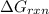 , we use the formula:
, we use the formula:
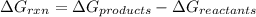
For the given chemical reaction:

We are given:
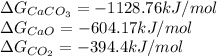
Now, to calculate
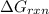 , we put the values in the above equation:
, we put the values in the above equation:
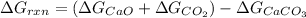
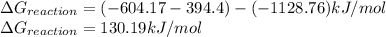
As, the value of
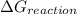 comes out to be positive, the reaction is said to be non-spontaneous reaction.
comes out to be positive, the reaction is said to be non-spontaneous reaction.