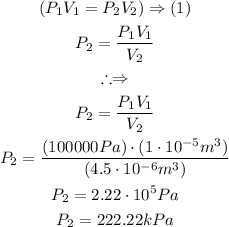Answer:
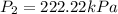
Step-by-step explanation: By using Gas Law, the new pressure can be calculated as follows:
Gas law states:
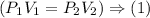
Using (1), and Identifying the knowns and unknowns, and plugging in (1) we get the following results:
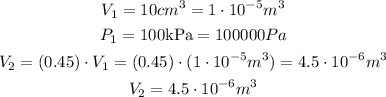
Finally, the New pressure is calculated as follows: