Answer:
Energy of photon,
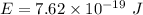
Step-by-step explanation:
It is given that,
The frequency of the photon,
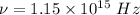
Value of Planck's constant,
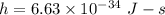
We have to find the energy of the photon. The energy of photon is given by the product of Planck's constant and the frequency of photon. It is given by :
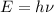
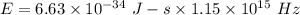
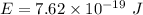
Hence, this the required solution for the energy of the photon.