Step-by-step explanation:
According to Bronsted-Lowry, acids are the species that give hydrogen ions whereas bases are the species that accept hydrogen ions.
A base on accepting a hydrogen ion results in the formation of conjugate acid. On the other hand, an acid on donating hydrogen ion results into the formation of a conjugate base.
For example,
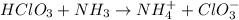
Here,
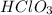 is the acid,
is the acid,
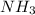 is the base,
is the base,
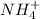 is the conjugate acid and
is the conjugate acid and
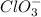 is the conjugate base.
is the conjugate base.
Therefore, we can conclude that in the given chemical reaction
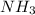 and
and
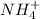 is the base-conjugate acid pair.
is the base-conjugate acid pair.