Answer:
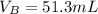
Step-by-step explanation:
Hello,
In this case, one uses the titration equation that comes from the moles equivalence during the neutralization:
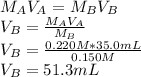
Such equality is used since the acid is monoprotic and the base has just one hydroxile allowing the moles to be equal at the equivalence point.
Best regards.