Answer:
To answer the question we will use the following equation:
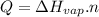
Where:
Q is the energy we need to vaporize the substance
Hvap is he enthalpy of vaporization
n is the number of moles
We first calculate the number of moles by using the molar mass of CH2Cl2:
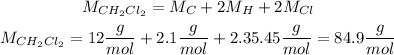
We calculate n (number of moles):
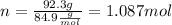
Now we calculate Q (energy)
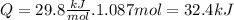
So the answer is 32.4kJ