An equilibrium constant is the ratio between the concentration of products and reactants, considering their stoichiometric numbers. In a general equation, for example:
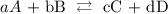
the equilibrium constant would be:
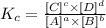
(where [C] = product C concentration, [D] = product D concentration etc.)
Considering that definition and the reaction given in the question, we could write the equilibrium constant as:
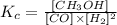
Note that the coefficient for both CH3OH and CO2 is 1, so we don't need to show it on the Kc formula.
Therefore, the numerator would be [CH3OH] and the denominator would have the terms [CO] and [H2]^2.