Answer: The correct answer is:
The volume and pressure of a gas are inversely proportional when temperature is constant.
Step-by-step explanation:
Boyle's Law states that pressure of gas is inversely proportional to the volume of the gas at constant temperature in Kelvins.
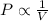 (At constant temperature)
(At constant temperature)
Where: P = pressure of the gas
V = Volume occupied by the gas