Answer:
The mass of the rubber ball is, 169.5 g
Explanation:
Using formula:
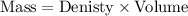 ......[1]
......[1]
As per the statement:
Isabella is bouncing a rubber ball that has a density of 1.5 g/cm3 . The rubber ball has a volume of 113 cm³.
⇒Volume = 113 cm³ and Density = 1.5 g/cm³.
Substitute these in [1] we get;
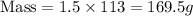
Therefore, the mass of the rubber ball is, 169.5 g