To calculate the density of an object, we must divide its mass by its volume.
For the case of the given penny, since it is cylindrical in form, then to solve for the volume, we have
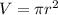
. Since the penny has a diameter of 1.9 cm, then it has radius of 1.9/2 = 0.95 cm.
Thus, its volume is
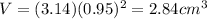
Now to get the density, we have
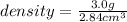
Therefore, the penny's density is
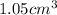
.