Answer:
It would take 0.1 minutes (approximately 6 seconds)
Step-by-step explanation:
Applying the Faraday's first law of electrolysis which states that,
m = ZIt
where m is the mass electroplated, Z is the electrochemical equivalence of the element, I is the current and t is the time taken.
Z of gold = 2.04352g/As
Thus,
t =

=
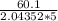
= 5.882 s
To determine the time in minute, divide by 60;
t = 0.0980minute
t = 0.1 minute