Answer:
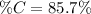
Step-by-step explanation:
Hello,
In order to compute the percent by mass of carbon in the ethene, C₂H₄, one applies the formula:
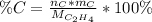
Whereas
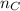 is the amount of carbon atoms into the hydrocarbon,
is the amount of carbon atoms into the hydrocarbon,
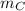 the carbon's atomic mass and
the carbon's atomic mass and
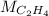 the molar mass of the ethene which is 28g/mol, thus:
the molar mass of the ethene which is 28g/mol, thus:
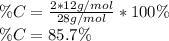
Best regards.