Answer: Option (A) is the correct answer.
Step-by-step explanation:
An isotope with an unstable nucleus which decomposes readily and results in the emission of radiation and a nuclear electron or helium nucleus achieves a stable nuclear composition is known as a radioactive isotope.
For example,
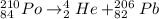
Here isotope of polonium decomposes in order to achieve a stable nuclear composition.
Thus, we can conclude that an atom is unstable best describes a radioactive isotope.