Answer: The correct answer is option A.
Explanation: Hydroxide ions are the poly-atomic ions which are generally released when a base is dissolved in water. The compound is base.
For the given options:
Option A: When base reacts with lipids, it results in the formation of soaps.
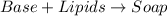
Option B: When base reacts with non-metal oxide, it results in the formation of salt and water.
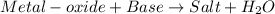
Option C: When acid reacts with metals, it results in the formation of salt and hydrogen gas.
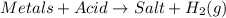
Option D: When acid reacts with metal carbonates, it results in the formation of salt, carbon dioxide and water.
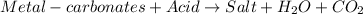
Hence, the correct answer is Option A.