What volume of a 0.0943 M H2SO4 solution, to three sig figs, is needed to neutralize 161.2 mL of a 0.0158 M LiOH solution given the following reaction?
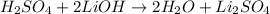
I was trying to use the following equation to find volume but im keep getting wrong answer
m1v1=m2v2