Answer: 3
Explanation: This is a radioactive decay and all the radioactive process follows first order kinetics.
Equation for the reaction of decay of
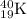 radioisotope follows:
radioisotope follows:
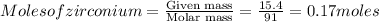
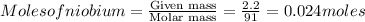
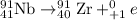
By the stoichiometry of above reaction,
1 mole of
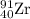 is produced by 1 mole
is produced by 1 mole
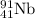
So, 0.17 moles of
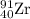 will be produced by =
will be produced by =
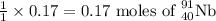
Amount of
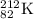
decomposed will be = 0.17 moles
Initial amount of
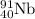 will be = Amount decomposed + Amount left = (0.17 + 0.024)moles =0.194 moles
will be = Amount decomposed + Amount left = (0.17 + 0.024)moles =0.194 moles
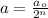
where,
a = amount of reactant left after n-half lives = 0.024
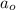 = Initial amount of the reactant = 0.194
= Initial amount of the reactant = 0.194
n = number of half lives= ?
Putting values in above equation, we get:
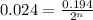
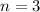
Therefore, 3 half lives have passed.