Answer:
The density of the aluminum block is 6.41 g per cm³ .
Explanation:
Formula
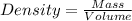
As given
An aluminum block has a mass of 82.0 g and a volume of 12.8 cm³.
Mass = 82.0 g
Volume = 12.8 cm³
Put in the formula
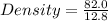
Density = 6.41 g per cm³ (Approx)
Therefore the density of the aluminum block is 6.41 g per cm³ .