Answer:
Q = 2926 kJ
Step-by-step explanation:
Given that,
Initial temperature of water,
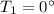
Final temperature of water,

Mass, m = 20 kg
To find,
Heat energy required to raise the temperature.
Solution,
Let Q is the heat energy required to raise the temperature. It is based on the concept of specific heat. The expression for the heat raised is given by :
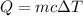
c is the specific heat of water,
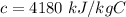
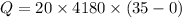
Q = 2926000 Joules
or
Q = 2926 kJ
So, the heat energy required to raise the temperature of water is 2926 kJ.