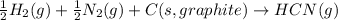Answer : The balanced chemical equation will be,
Explanation :
Standard formation of reaction : It is a chemical reaction that forms one mole of a substance from its constituent elements in their standard states.
The hydrogen cyanide (HCN) is formed by the combination of hydrogen, carbon and nitrogen.
The balanced chemical equation for the standard formation reaction of gaseous hydrogen cyanide will be,