Answer: The bond angle of a trigonal planar molecule is 120 degrees.
Step-by-step explanation:
Boron trifluoride is trigonal planar, that is, boron atom is located at the center and the three fluorine atoms are attached to it in the form of an equilateral triangle.
It is known that a trigonal planar molecule has a bond angle of 120 degrees.
The hybridization of
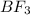 is
is
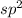 .
.