Answer : The enthalpy change for this reaction is, -900.8 KJ
Solution :
The balanced chemical reaction is,
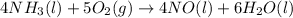
The expression for enthalpy change is,
![\Delta H=\sum [n* \Delta H_f(product)]-\sum [n* \Delta H_f(reactant)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/pvgo4z4xrppf3xk4s9yzphzrjz2gc51k03.png)
![\Delta H=[(n_(H_2O)* \Delta H_(H_2O))+(n_(NO)* \Delta H_(NO))]-[(n_(NH_3)* \Delta H_(NH_3))+(n_(O_2)* \Delta H_(O_2))]](https://img.qammunity.org/2018/formulas/chemistry/high-school/ihsplkbu2s1ce2xzb26y6pgimkmwps9btl.png)
where,
n = number of moles
Now put all the given values in this expression, we get
![\Delta H=[(6* -241.8)+(4* 91.3)]-[(4* -46.2)+(5* 0)]\\\\\Delta H=-900.8KJ](https://img.qammunity.org/2018/formulas/chemistry/high-school/doojnymbjv2gp0m8au72si849yyww43nra.png)
Therefore, the enthalpy change for this reaction is, -900.8 KJ