Answer:- Precipitate will form.
Solution:- A precipitate is formed if the ionic product is greater than solubility product, (
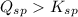 )
)
0.10 L of calcium chloride and 0.50 L of sodium fluoride are added. So, total volume of the solution = 0.10 L + 0.50 L = 0.60 L
We get the calcium ions from calcium chloride as:
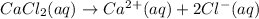
From this equation, there is 1:1 mol ratio between calcium chloride and calcium ion. So, the initial concentration of calcium ion will be same as of calcium chloride. As the volume is additive, the final concentration of calcium ion is calculated using the dilution equation:
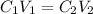
It could also be written as,
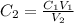
Let's plug in the values in it:
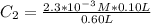
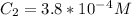
Similarly there is 1:1 mol ratio between sodium fluoride and fluoride ions. we can calculate the final concentration of Fluoride ion as:
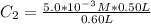
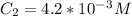
Now, we will calculate the ionic product as:
![Q_s_p=[Ca^2^+][F^-]^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/h4djjzdvrtksjvn91kou0bpn5yk2tsykox.png)
![Q_s_p=[3.8*10^-^4][4.2*10^-^3]^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/fz1w65cib3bflt4x6ors5n3ikxl4uzjxo1.png)
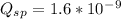
Given solubility product is
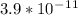 which is less than the calculated value of ionic product. (
which is less than the calculated value of ionic product. (
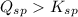 ) .
) .
Hence. the precipitate will form.