Answer:when the rates of the forward and reverse reactions are equal
Step-by-step explanation:
Dynamic Equilibrium : It is a reaction which never stops. But in this type of equilibrium concentration of products and reactants doesn't get alter with the time.The rate of reaction going in a forward direction is equal to the rate of reaction going in a backward direction.
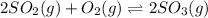
Hence, the given chemical system reach dynamic equilibrium when the rates of the forward and reverse reactions are equal