Answer: Yes it will sink.
Step-by-step explanation
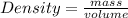
If the density of a substance in less than of the another substance then the substance with less density will float in substance with higher density or vice versa.
Density of iron block:
mass of iron = 213 g
Volume of block = length× breadth×height=
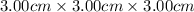
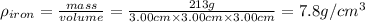
Density of the water =
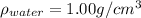
The
 , density of iron block is more than of the water which means that iron block will get sink in the water.
, density of iron block is more than of the water which means that iron block will get sink in the water.
So, yes it will sink.