Answer: The correct answer is Option A.
Step-by-step explanation:
Reducing agent is defined as the agent which helps the other substance to get reduced and it itself gets oxidized. These agents undergo oxidation reaction.
Oxidation reactions are the reactions in which a substance looses its electrons to form a positive ion.
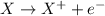
Hence, the correct answer is Option A.