Answer: The reaction
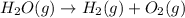 would have increase in entropy.
would have increase in entropy.
Explanation: Entropy is the measure of degree of randomness or disorder of a system. Thus a system with less disorder has less entropy and a system with more disorder has more entropy. The balanced chemical equation will be:
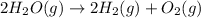
The given reaction leads to formation of 3 moles of gaseous products from 2 mole of gaseous reactant. Thus the given reaction leads to increase in moles and thus increase in disorder and increase in entropy.