Answer : Option E) 50 grams.
Explanation : According to the solubility curves the compound
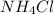 to dissolve at 50 °C in 100 mL of water will need 50 grams of the compound. It is clearly indicated in the graph which is marked with red that at 50°C approximately 50.4 grams of the compound
to dissolve at 50 °C in 100 mL of water will need 50 grams of the compound. It is clearly indicated in the graph which is marked with red that at 50°C approximately 50.4 grams of the compound
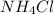 will be needed to dissolved in 100 mL of water to form a solution.
will be needed to dissolved in 100 mL of water to form a solution.